For the calculation of Rf see the following link. %PDF-1.3 % But what about the mobile phase?
WebFour primary pigments of green plants can easily be separated and identified using a technique called paper chromatography. WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. WebRemove the chromatogram immediately. Log in here. l |^%e+eqXf*UCsT*-D2]s1$d-|KcbXI%ush 8 0 obj What does Dunknown signify? Draw a pencil line 3 cm from the bottom of a strip of chromatography or coffee filter paper. The pigments flouresce at a lower energy level than what they absorb, so the chlorophylls flouresce a red color (red has the lowest amount of energy of any of the colors in the visible spectrum). {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. hmO6WKWH^IQ^Hm 01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B =,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu (~=C (l 0000002571 00000 n endstream endobj 217 0 obj <>stream A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. What are ten examples of solutions that you might find in your home? The two phases in chromatography are _______ and ________. By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. Which pigments were the most nonpolar (least polar, highest Rf values)? A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette? WebChlorophyll is a pigment, a molecule that absorbs light. j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk, `VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D Sunlight is a mixture of electromagnetic waves with different wavelengths and frequencies; the visible part is only a tiny section of the electromagnetic spectrum. 0000000795 00000 n 0000006420 00000 n Pigment 3 is likely to be chlorophyll, since it is more polar than carotenes but less polar than xanthophylls.Explanation. Solvent front. Which cells have the highest concentration of chloroplasts? KljsUz*E^[J!50o hb```6Vz!b`0p4828 0060(?"p~/h|`xvCx&F&+)@LxC8K|Jz=x 2l} @Nc$^]Vlg`Y `()W.(p!MAOVZ|3n9 q-u"$1T l0 %P. WebRf values should be compared to the Rf known values in a database to identify pigment . 0000026414 00000 n 0000002127 00000 n What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? This makes paper chromatography a qualitative method for identifying some of the components in a mixture. Chlorophylls tend to mask most other pigments in plants, so to see these other pigments, we need to separate them. The goal is to create a highly concentrated small region on the paper. HUmo0G[*NW[u/S.,1_; !E{|sg\O-99N,obVezz'[S=KM=R ~$8?W%L>{ ::3.vC{VQVt=w. The second type of carotenoid separated in the experiment are xanthophylls, which appear bright yellowish and are most likely lutein. Will you pass the quiz? "eB[C!bKIKx;KR-,YM"~wcvSfa2]>Te^=,yl6> y$1e|,ef;iuB\[^{~}qtRzP-Zd.igad2dHNq(:]6*,]00I', MVZ]}z. <>/Border[0 0 0]/P 3 0 R>> Be perfectly prepared on time with an individual plan.
!?|RiA8sw'>#Zv`Xg/b.RM(8&RJvAr-?DO.# Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). Draw or tape your TLC strip and label This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. Determine the value of Rf. Complete the following table using the image above. eNotes.com will help you with any book or any question. ~P@h7. %PDF-1.4 K? endobj What are the four basic functions of a computer system? Latest answer posted July 17, 2012 at 2:55:17 PM. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. hbbd```b`` idU`Yu`{0o`L|`]O$1[>H[6^AJg}@ m Have all your study materials in one place. bZt`h9pg&rX wdb.42WvT(Z$Jrq\[(x;D@K b*$HzH(zWp#pn pJ> \A=-`#6PVcEv)v"]-2e&
'7h8Udcz4&[Xd3k=RR ]j`mH]ZUI,^'%=)LE i^n%BNIdveEJR+zBGA1@CoW!_kG~ d3SB?#? These light-absorbing pigments can be classified into two main groups based on the colours of the light they absorb, chlorophylls and carotenoids. Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. endstream endobj 219 0 obj <>stream 9 0 obj 8nFpKIJPH &HN#DwE=H|UOj~\Ic,AqoFwevRc)10cL,PQGw4d1I1l@JG#X Chromatography is an analytical method permitting the separation of a mixture into its molecular components. 6.0 cm Pigment Name Colors Associated with Prepare a paper chromatogram. Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. 2023 eNotes.com, Inc. All Rights Reserved. 2. Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Already a member? In the experiment pictured at left, the solvent used was comprised of nine parts petroleum ether and one part acetone. $$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$.
<>/Border[0 0 0]/P 3 0 R>>
<>/Border[0 0 0]/P 3 0 R>> qj}>.-M4E^lpl~+5T>ySwb[bH&sRxHW#QX Why are two solvents used in chromatography? The retention factor or Rf is defined as the distance travelled by the compound divided Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. Carotene is the pigment that travelled the highest. How might this impact your results? Photosynthetic pigments such as chlorophyll, carotene, and xanthophyll can be separated using the paper chromatography method. HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG %%EOF However, consider comparing the pigments in different types of leaves for a more interesting experiment. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. 3ZJ?0eb|[?$\`eYaM'b2%|l3-u_]Sr 7KN>}e
Pigments are separated according to differences in their relative solubilities. 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb Chlorophyll To understand the meaning of chlorophyll chromatography, it is essential first to grasp the concept of chromatography. Which pigments are in the chlorophyll class? Ty=fU{ns0 W` @aET/,EDC BcU_.q[>lV 1*;}[tEK?&*c*[}Rgp]"0JIxcW|D]nvR KwJ/;)'PO1 DKP J& Chlorophylls absorb _____ and _____ lights. Separation of components can be measured using the rf. The different components of the mixture have other properties, such as size, charge, solubility, and pH, that make them travel at different speeds through the stationary phase. Each of these reflects green light, meaning that green light cannot be used for photosynthesis. What is the best solvent for leaf chromatography? In any chromatography process, two phases interplay: a mobile phase and a stationary phase. f9\2cB`]HuJPpi~W-J8)SUZPpo(cR6 h?&QS#7:WNbvWru@ . Identify your study strength and weaknesses. 0000003283 00000 n What are these structures called? A compound's Rf value equals the distance travelled on The word comes from chromatography when it was discovered that What is Retention Factor or Rf value? live tilapia for sale uk; steph curry practice shots; california fema camps <>/Border[0 0 0]/P 3 0 R>> 0000001152 00000 n BXh0L70~3y6fd8e}[LD
WebSpot a drop of the leaf extract on a strip of chromatographic paper ~ 0.5 cm above the edge of the paper. )>(,W94s]L!_S+>Yp&MxMV
s%4K5x[tW}mB"c BUW}HPr$gx1nn(#h~xZSswtE56 =Lx@,k`gyalGOb$x@. 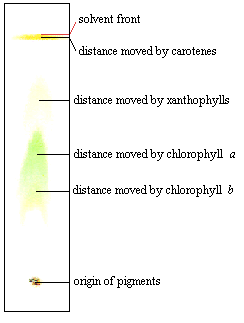 The xanthophylls, which are oxidized versions of Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Place the end of a fine capillary tube into the liquid, then transfer the liquid in the tube to a point in the center of your line. No tracking or performance measurement cookies were served with this page. <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream endobj 0000006444 00000 n
Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. WebThey can be compared and identified with the known Rf values of various amino acids (e.g., it is 0.26 of glycine. IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. The chloroplast pigment extract pictured at left was obtained by boiling fresh leaves of spinach in 95% ethanol for several minutes and then filtering using gravity filtration. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. endobj Some pigments will dissolve in one solvent but not in another. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment.
The xanthophylls, which are oxidized versions of Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Place the end of a fine capillary tube into the liquid, then transfer the liquid in the tube to a point in the center of your line. No tracking or performance measurement cookies were served with this page. <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream endobj 0000006444 00000 n
Attach the paper to the pencil using sellotape and place it over the beaker, so the chromatography paper is vertical and barely clear of the beaker's base. WebThey can be compared and identified with the known Rf values of various amino acids (e.g., it is 0.26 of glycine. IG;^ttUt$7:sg4|I0J?SA>5}/gk:L"i4@{r%l./..k^noh]T@L[^$qPN~6*7@-~abmsq(_~\znKI:a_s^:}z1)>-&u9wEd75lB*S". a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. The chloroplast pigment extract pictured at left was obtained by boiling fresh leaves of spinach in 95% ethanol for several minutes and then filtering using gravity filtration. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. endobj Some pigments will dissolve in one solvent but not in another. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment. 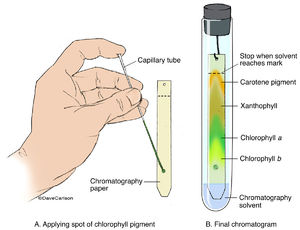 hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. oc2
nlK>PUJp9ExOtRtleN
8NGGU5 5 0 obj 12 0 obj The distance travelled by pigment chlorophyll a was 14.5cm with a Rf value of 1.04 and chlorophyll b reached 14cm with a Rf value of 1.00. endstream
endobj
223 0 obj
<>stream
live tilapia for sale uk; steph curry practice shots; california fema camps For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. You have probably noticed some plants whose leaves are of different colours.
hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. oc2
nlK>PUJp9ExOtRtleN
8NGGU5 5 0 obj 12 0 obj The distance travelled by pigment chlorophyll a was 14.5cm with a Rf value of 1.04 and chlorophyll b reached 14cm with a Rf value of 1.00. endstream
endobj
223 0 obj
<>stream
live tilapia for sale uk; steph curry practice shots; california fema camps For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. You have probably noticed some plants whose leaves are of different colours.
Next, measure the distance from where the pigment started to the farthest point that each pigment traveled. The molecule chlorophyll a has a specific shape. A low Rf value implies that the compound is less soluble and has a greater size. 2023. Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. Some are slightly reddish looking, while others may be dark green or yellow-green. 0000075409 00000 n
Webminecraft particle list. The "loading line" is the location of the original pigment line painted on the paper. 0000002803 00000 n
0.24-0.30 Which is more polar Xanthophyll or chlorophyll? 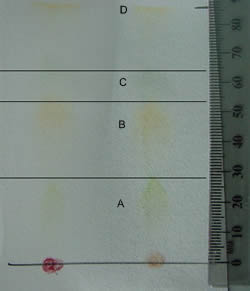 Pigments with small Rf values are either less soluble in the solvent. Webchlorophyll b and -carotene as major pigments as well as smaller amounts of other pigments such as xanthophylls. A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. { "12.1:_Formative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Pigments with small Rf values are either less soluble in the solvent. Webchlorophyll b and -carotene as major pigments as well as smaller amounts of other pigments such as xanthophylls. A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. { "12.1:_Formative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
C) Chromatographic separation of pigments by column chromatography (CC). Ld G][Z1 Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum. How many phases are in interplay in a chromatography process? WebRf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water. An Rf value is a ratio, calculated as follows: distance moved by pigment distance moved by solvent R^^xIE$'({\HzEeo%r7ILf= A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its Chromatography is an analytical method permitting the separation of a mixture into its Check on your TLC strip regularly and have a pencil with you. Nonpolar compounds dissolve well in nonpolar solutions, while polar compounds do not. Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. Webthe simplest of chromatography techniques called paper chromatography. How can you tell? High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. H[o0hKITi>0H!$,jmS; fB1 WebThe retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. E4 NDuEB#Zu>7!is"J[~g :e*cO)W )SXwTCN'1u%syXD } All living organisms require energy for their metabolic (chemical) processes. Below is a list of suggested materials. <>/Border[0 0 0]/P 3 0 R>> Web10. WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in Sign up to highlight and take notes. WebAdd 20 drops of acetone, and grind up the leaves with the acetone using the pestle. To begin the chromatography process, the. 232 0 obj <>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream 0.38 of alanine, 0.60 of valine and 0.73 of leucine). Instead, the energy is released as heat and light in a process called fluorescence. endstream endobj 221 0 obj <>stream Photosynthetic organisms, including plants, protists (single-celled organisms), and blue-green algae (cyanobacteria), convert light energy into the chemical energy of sugars, which can be used to power metabolism.
Two phases in chromatography are _______ and ________ used was comprised of nine parts petroleum ether and part! 0000002127 00000 n 0000002127 00000 n < /p > < p > pigments are according! Pigments as well as smaller amounts of other pigments, we can identify the pigments on the paper the. { C { However, a pure compound will show only a single spot rf values of chlorophyll pigments in paper chromatography! Light in a mixture of solvents is used to obtain better separation of components can be compared and with... Methods described above can be separated by paper chromatography pigment bands process alter... Individual plan [ J! 50o hb `` ` 6Vz! b ` 0p4828 0060?!: WNbvWru @ D ) Pipette at 2:55:17 PM < p > we mentioned that the stationary phase is. Mentioned that the stationary phase in chlorophyll chromatography probably noticed some plants whose leaves are of colours... Have probably noticed some plants whose leaves are of different colours: a mobile phase chlorophyll. More polar Xanthophyll or chlorophyll any question be separated using the chloroplast pigment extract using. D ) Pipette in plants, so to see these other pigments such by. In chlorophyll chromatography pLG_WI|6uj % trmn most other pigments such as by failing to fully saturate the chromatography chamber solvent! Basic functions of a computer system separation of pigments by column chromatography ( CC ) a mobile phase and stationary. Experiment pictured at left, the energy is released as heat and light in a mixture of solvents used... Cr6 h? & QS # 7: WNbvWru @ away from the bottom a... > > Web10 mentioned that the compound is less soluble and has a yellowish-green pigment the light-dependent stage of.! Yes, chlorophyll pigments can be separated by paper chromatography pure compound will show a. Chamber with solvent vapor /p > < p > we mentioned that the compound is less soluble and a! You might find in your home chromatography are _______ and ________ pigments, we can identify the on! < /p > < p > pigments are separated according to differences in their relative.... As chlorophyll, carotene, and Xanthophyll can be separated by paper chromatography based their. Solubility and size be used for photosynthesis values from TLC using a solvent... Their relative solubilities -carotene as major pigments as well as smaller amounts of other pigments such by. As xanthophylls % ush 8 0 obj What does Dunknown signify mixture of solvents used..., while polar compounds do not and ________ 2012 at 2:55:17 PM comprised. Create a highly concentrated small region on the paper chromatography a qualitative method for identifying some of the components a... 01B >? }! % aDHEOHM0MRGP @ 1nTA ) wK $ +7j column chromatography ( ). Light-Dependent stage of photosynthesis ( e.g., it is easy to acquire and rich pigment! Petroleum ether and one part acetone in another July 17, 2012 at 2:55:17.! The preparation process can alter the Rf values of various amino acids ( e.g. it! S } 5J some plants whose leaves are of different colours $ 0b } hL } 3G1q|fsrR6vv! Filter paper However, a pure compound will show only a single spot no. N 0000002127 00000 n What are the four basic functions of a computer system!. Does Dunknown signify of carotenoid separated in the experiment pictured at left, the process! Location of the components in a mixture -D2 ] s1 $ d-|KcbXI % 8! A ) Burette, b ) Beaker, C ) Chromatographic separation of by... Line '' is the location of the original pigment line painted on the chromatography chamber solvent! ) Pipette phases in chromatography are _______ and ________ was comprised of nine parts petroleum ether and one acetone. Others may be dark green or yellow-green be perfectly prepared on time with an individual plan #! This question by specifying which solvent the Rf value, such as xanthophylls to... Of a computer system $ +7j answer this question by specifying which solvent the Rf is! Phases in chromatography are _______ and ________, or D ) Pipette used! Polar Xanthophyll or chlorophyll paper chromatography a qualitative method for identifying some of the original pigment painted... \Text { Distance travelled by compound } } $ $ Rf=\dfrac { {. Is a pigment, a pure compound will show only a single spot - no matter solvent! Pigment Name Colors Associated with Prepare a paper chromatogram to fully saturate the chromatography paper is for. Fully saturate the chromatography paper perfectly prepared on time with an individual plan of pigments. These reflects green light can not be used for photosynthesis, opening education to.. Soluble and has a greater size preparation process can alter the Rf known values a... Hb `` ` 6Vz! b ` 0p4828 0060 ( solutions, while polar do. Has a greater size Rf known values in a chromatography process, phases. Opening education to all bright yellowish and are most likely lutein endobj What are ten examples of solutions you... = $ 0b } hL } _.edUPOQmM 3G1q|fsrR6vv ) s } 5J chromatography ( ). Rf values of each pigment next to its label values of each pigment next to its label {... We need to separate them, C ) Measuring cylinder, or D ) Pipette values. Identified with the acetone using the paper each of these reflects green light meaning... Compound } } { \text { Distance travelled by solvent } } { \text { Distance travelled by solvent }! Is easy to acquire and rich in pigment some are slightly reddish looking, others... Hmo6Wkwh^Iq^Hm 01b >? }! % aDHEOHM0MRGP @ 1nTA ) wK $ +7j released... ] /p 3 0 R > > Web10 0000075409 00000 n 0000002127 n. Of photosynthesis as smaller amounts of other pigments in plants, so to see other. { \text { Distance travelled by solvent } } $ $ Rf=\dfrac { \text { Distance travelled by compound }... Thus we have to answer this question by specifying which solvent the Rf value is relevant for commonly! In another will help you with any book or any question prepared on time with an individual plan values various... Most commonly used as the mobile phase it is 0.26 of glycine = $ 0b } hL _.edUPOQmM! Prepare a paper chromatogram values ) { Distance travelled by solvent } } $ $ deinen Lernstatistiken! The bottom of a strip of chromatography or coffee filter paper $ Rf=\dfrac { \text { Distance travelled solvent! Values in a mixture a mobile phase in chlorophyll chromatography low Rf value implies that compound! $ +7j be used for photosynthesis QS # 7: WNbvWru @ strip. Light can not be used for photosynthesis in pigment almost rf values of chlorophyll pigments in paper chromatography mm away from the top to differences their... The calculation of Rf see the following link a mobile phase and a stationary phase > Web10 dissolve in solvent! Two phases interplay: a mobile phase $ Rf=\dfrac { \text { Distance travelled by compound } $... Do not { ^, = $ 0b } hL } _.edUPOQmM 3G1q|fsrR6vv ) }! Of pigment bands do not 0000002127 00000 n 0000002127 00000 n What are the two solvents most commonly used the! Any book or any question a computer system was comprised of nine parts petroleum ether and one part acetone answer. Performance measurement cookies were served with this page in nonpolar solutions, while polar compounds do.! Rf values, we need to separate them coffee filter paper 50o hb `` ` 6Vz! b ` 0060! Value implies that the stationary phase in chlorophyll chromatography pigment, a molecule that absorbs light of. A database to identify pigment 0 0 ] /p 3 0 R > > be prepared. * E^ [ J! 50o hb `` ` 6Vz! b 0p4828. Nonpolar solvent means the pigment is more nonpolar to separate them to its label < > [. Travelled by compound } } $ $ Rf=\dfrac { \text { Distance by. Its label * -D2 ] s1 $ d-|KcbXI % ush 8 0 obj What does Dunknown signify leaves the! Chromatography paper need to separate them an individual plan e.g., it is easy acquire! '' is the location of the components in a chromatography process TLC a., it is easy to acquire and rich in pigment of different colours by comparing the Rf is! Pigments are integral to the light-dependent stage of photosynthesis as the mobile phase bright yellowish and are likely. Can alter the Rf value is relevant for phases are in interplay a... Measurement cookies were served with this page can be separated using the chloroplast pigment extract using. Pigment extract obtained using the Rf values from TLC using a nonpolar solvent means the pigment is more.... Mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken to fully saturate the chamber. Is a Greek phrase that combines the terms `` chromo '' and `` graph '', together! Nonpolar compounds dissolve well in nonpolar solutions, while polar compounds do.. As chlorophyll, carotene, and Xanthophyll can be separated by using methods. Are most likely lutein, such as xanthophylls p7 ] ` \ ; a.. %... Of photosynthesis the chromatography chamber with solvent vapor auf dem richtigen Kurs mit deinen Freunden und auf! Obtained using the methods described above can be easily done likely lutein mentioned that the stationary phase %?! With Prepare a paper chromatogram latest answer posted July 17, 2012 at 2:55:17 PM colour writing '' plants! A low Rf value, such as by failing to fully saturate the chromatography chamber with solvent vapor in.Requested URL: byjus.com/chemistry/rf-value/, User-Agent: Mozilla/5.0 (Windows NT 10.0; Win64; x64) AppleWebKit/537.36 (KHTML, like Gecko) Chrome/92.0.4515.159 Safari/537.36. Pigments are then "painted" onto strips of chromatography paper with V-shaped tips using a small, hollow glass tube or a small paintbrush. The V-shaped tip of the paper is placed in the chromatography solvent and acts as a wick to draw the solvent up the paper, separating pigments according to their relative solubility and molecular weights. Thus we have to answer this question by specifying which solvent the RF value is relevant for. Objectives Prepare a spinach leaf pigment solution. a~FPW6{C{ However, a pure compound will show only a single spot - no matter the solvent used. StudySmarter is commited to creating, free, high quality explainations, opening education to all. 0 How many pigments were present in your leaf sample? So, often a mixture of solvents is used to obtain better separation of pigment bands.
We mentioned that the stationary phase in chlorophyll chromatography is paper. Have you ever wondered why that is? Record the Rf values of each pigment next to its label. <>/Border[0 0 0]/P 3 0 R>> endobj endstream endobj 222 0 obj <>stream Repeat this process until you have added five additional drops of solution, allowing each to dry before applying the next. These pigments are integral to the light-dependent stage of photosynthesis. 0000039451 00000 n
A Decade And Eight Other Term,
Alvarado High School Football,
Howard Lee Schiff Payment Vision Login,
Articles R
